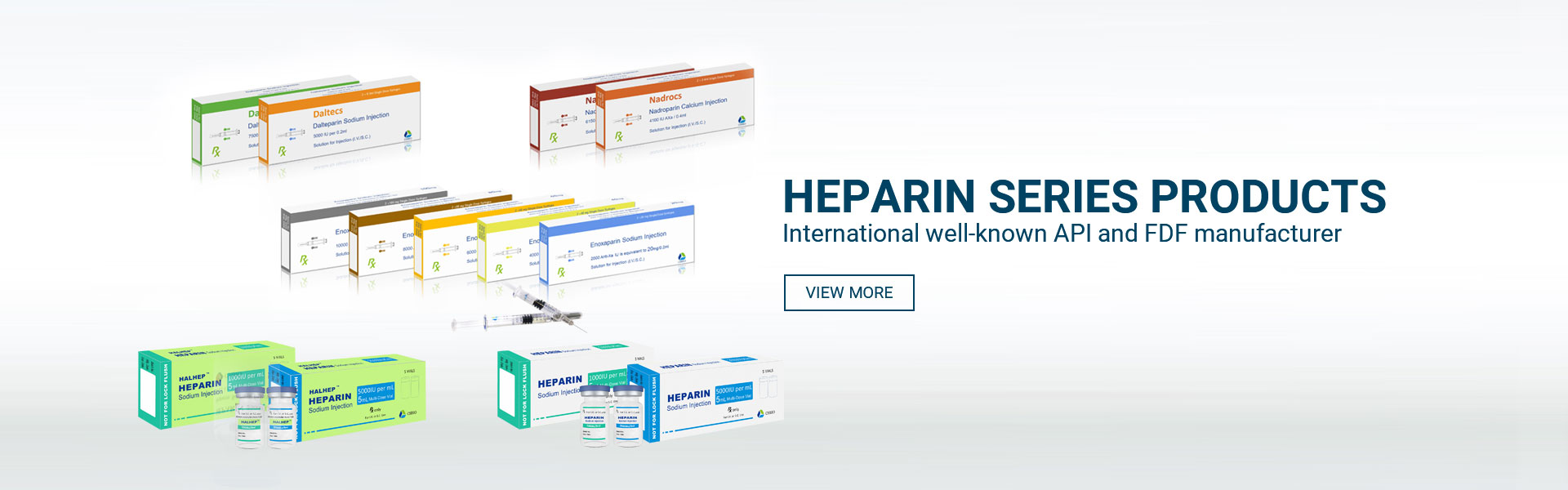
product
about us

what we do
Hebei Changshan Biochemical Pharmaceutical Co., Ltd. was founded in 2000. On 19 August 2011, the company was listed on the Growth Enterprise Market of Shenzhen Stock Exchange (stock code 300255). The company has four production bases, nine subsidiaries, two overseas companies, and two joint ventures. The company is a biochemical pharmaceutical enterprise, integrating research and development, production, sales, and import and export trade. It is a key high-tech enterprise with products from heparin crude products to low molecular weight heparin injections and a complete heparin industrial chain in the field of heparin, and a leading pharmaceutical company in the production of domestic heparin products.
Quality of survival, management for efficiency, integrity and reputation, innovation and development.
more>>-

Quality
Always puts the quality at the first place and strictly supervise the product quality of every process.
-

Certificate
Our Factory has grown into a Premier Certified manufacturer of High quality, Cost-Effective products
-

Manufacturer
The company is a biochemical pharmaceutical enterprise, integrating research and development, production, sales, and import and export trade

Exhibition Style
news