China Factory for Heparin Anticoagulant – Dalteparin Sodium – CSBIO
China Factory for Heparin Anticoagulant – Dalteparin Sodium – CSBIO Detail:
INDICATION:
Dalteparin Sodium belongs to a group of medicines called low molecular weight heparins or antithrombotics, which help prevent the formation of blood clots by thinning the blood.
• Dalteparin Sodium is used to treat blood clots (venous thromboembolism) and to prevent their recurrence. Venous thromboembolism is a condition where blood clots develop in the legs (deep vein thrombosis) or the lungs (pulmonary embolism), e.g. after surgery, prolonged bed-rest or in patients with certain types of cancer.
• Dalteparin Sodium is also used to treat a condition known as unstable coronary artery disease. In coronary artery disease the coronary arteries (blood vessels to the heart) are furred up and narrowed by patches of fatty deposits.
• Unstable coronary artery disease means that a furred up bit of the artery has ruptured and a clot has formed on it, reducing the flow of blood to the heart. Patients with this condition may be more likely to go on to have a heart attack without treatment with blood thinning drugs such as Dalteparin Sodium.
CHARACTERS:
Dalteparin sodium has the most ideal molecular weight distribution, and has both anticoagulant efficacy and safety. The molecular weight distribution of dalteparin sodium is the most concentrated, the antithrombotic activity is the strongest, the low molecular fragments are less, the drug accumulation is less, the polymer fragments are less, the binding rate with platelets is low, the incidence of HIT is low, and the bleeding risk is small.
It is safer for special groups:1. Dapaparin is the only low-molecular-weight heparin approved by the US FDA for safe use in the elderly. 2. Dalteparin sodium is the only low-molecular-weight heparin that has no significant accumulation in patients with renal impairment.
COMPANY ADVANTAGE
1.full product Chains:
We have the full product Chains, which starts from the Porcine mucosa with be processed to Crude heparin, Heparin sodium API that could be depolymerized to the API of Enoxaparin sodium, Dalteaprin sodum and Nadroparin calcium. We could control the product from the starting material and make sure the tracebility which is required by the regulated market. At present, we have our own crude heparin workshop, which could help us to not only control the cost bust also assure the quality of our product.
2.APIs’ Production Lines:
Facilities and Equipment Systems
There are dedicated production workshops respectively for Heparin API, Enoxaparin Sodium API and Dalteparin Sodium&Nadroparin Calcium API. These three workshops are all established and have their respective dedicated production equipments, HVAC system and purify water system, which could avoid the cross contamination.
3.Product qualification
ForDalteparin Sodium API : we have passed the followings audits, US-FDA, CFDA, Germany authority, EIR-LETTER, Germany and China GMP are available.
- Passed CFDA GMP in July 2014 & April 2019.
- Passed US FDA inspection in April 2015 .
- Passed Germany GMP in September 2015
Production capacity is enough:Dalteparin Sodium API: 3,000 kg
4.We basically estsabilshed GMP Six-system that can comply with the standard of EU GMP,US FDA CGMP and Chinese GMP which could make sure our product comply with the international quality systems.
5.The company is located in the Zhengding area of China (Hebei) Pilot Free Trade Zone, close to Shijiazhuang Airport and the high-speed railway station, with a good transportation location. On August 26, 2019, the State Council issued the “Overall Plan for China (Hebei) Pilot Free Trade Zone”. The Zhengding area focuses on the development of bio-medicine, international logistics and other industries. We will strive to build the company into a leading enterprise in the domestic heparin industry.
6.Main market: Italy, Russia, Ukraine, Belarus, Indian, Korean, Argentina, Turkey, Iran and so on.
7.Payment: TT in advance
Delivery details: within in 30days after confirmed the order by air
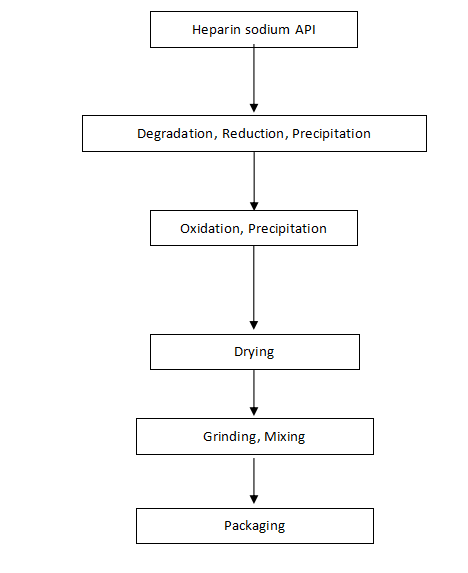
Manufacturing Process Flow Chart of Dalteparin Sodium
Dalteparin Sodium
|
Tested item |
Acceptance criteria |
|
Characters |
white or almost white powder |
|
Solubility |
Free soluble in water |
|
Identification |
A. 13C-NMR Spectrum should be similar to the reference spectrum. |
|
B. The mass-average relative molecular mass ranges between 5600 and 6400 |
|
|
pH |
5.5~8.0 |
|
Appearance of solution |
Clear; Not more intensely coloured than reference solution Y5. |
|
Nitrite |
Not more than 5ppm |
|
Nitrogen |
1.5% ~ 2.5% |
|
Sodium |
10.5% ~ 13.5% |
|
Boron |
≤1ppm |
|
Molar ratio of sulfate ions to carboxylate ions |
≥1.8 |
|
Loss on drying |
≤5.0% |
|
Heavy metals |
≤30ppm |
|
Potency of anti-factor Xa activity |
110~210AXaIU/mg (dried product) |
|
Potency of anti-factor IIa activity |
35~100AIIaIU/mg (dried product) |
|
The ratio of anti-factor Xa activity to anti-factor Iia activity |
1.9~3.2 |

Product detail pictures:

Related Product Guide:
Science, simplified:How vaccines work against COVID-19
Assume full duty to satisfy all demands of our clients; reach steady advancements by marketing the development of our purchasers; grow to be the final permanent cooperative partner of clientele and maximize the interests of customers for China Factory for Heparin Anticoagulant – Dalteparin Sodium – CSBIO , The product will supply to all over the world, such as: Barcelona, Cannes, Denmark, The company attaches great importance to product quality and service quality, based on the business philosophy "good with people, genuine to whole world, your satisfaction is our pursuit". we design products, According to customer's sample and requirements, to meet the needs of the market and offer different customers with personalized service. Our company warmly welcomes friends at home and abroad to visit, to discuss cooperation and seek common development!
As an international trading company, we have numerous partners, but about your company, I just want to say, you are really good, wide range, good quality, reasonable prices, warm and thoughtful service, advanced technology and equipment and workers have professional training, feedback and product update is timely, in short, this is a very pleasant cooperation, and we look forward to the next cooperation!






