2020 China New Design Heparin Sodium Solution - Nadroparin Calcium – CSBIO
2020 China New Design Heparin Sodium Solution - Nadroparin Calcium – CSBIO Detail:
INDICATION:
In surgery, used in moderate or high risk cases of venous thrombosis to prevent venous thromboembolic disease.
Treatment of deep vein thrombosis.
Combined with aspirin for the acute phase of unstable angina and non-Q-wave myocardial infarction.
Prevent the formation of blood clots during cardiopulmonary bypass during hemodialysi
COMPANY ADVANTAGE
1.full product Chains:
We have the full product Chains, which starts from the Porcine mucosa with be processed to Crude heparin, Heparin sodium API that could be depolymerized to the API of Enoxaparin sodium, Dalteaprin sodum and Nadroparin calcium. We could control the product from the starting material and make sure the tracebility which is required by the regulated market. At present, we have our own crude heparin workshop, which could help us to not only control the cost bust also assure the quality of our product.
2.APIs’ Production Lines:
Facilities and Equipment Systems
There are dedicated production workshops respectively for Heparin API, Enoxaparin Sodium API and Dalteparin Sodium&Nadroparin Calcium API. These three workshops are all established and have their respective dedicated production equipments, HVAC system and purify water system, which could avoid the cross contamination.
3.Product qualification
For nadroparin Calcium API : we have Germany and China GMP.
4.Production capacity is enough:Nadroparin Calcium API: 3,000 kg
5.We basically estsabilshed GMP Six-system that can comply with the standard of EU GMP,US FDA CGMP and Chinese GMP which could make sure our product comply with the international quality systems.
6.The company is located in the Zhengding area of China (Hebei) Pilot Free Trade Zone, close to Shijiazhuang Airport and the high-speed railway station, with a good transportation location. On August 26, 2019, the State Council issued the “Overall Plan for China (Hebei) Pilot Free Trade Zone”. The Zhengding area focuses on the development of bio-medicine, international logistics and other industries. We will strive to build the company into a leading enterprise in the domestic heparin industry.
7.Main market:Ukraine, Russia, Indian and so on.
8.Payment: TT in advance
Delivery details: within in 30days after confirmed the order by air
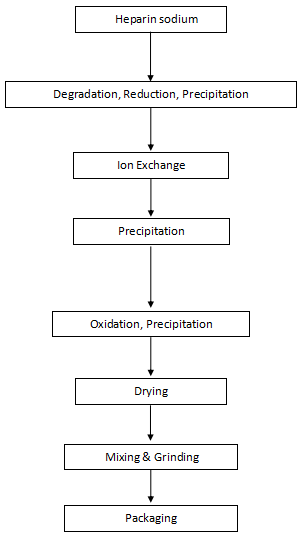
Flow Chart of Nadroparin Calcium
Nadroparin Calcium (EP)
|
Tests |
Specification |
|
Description |
White or almost white powder, hygroscopic. |
|
Solubility |
Freely soluble in water. |
|
Identification |
13C-NMR-spectrum of substance to be examined should correspond with the reference standard spectrum of Nadroparin calcium. The ratio of anti-factor Xa activity to anti-factor Ⅱa activity is between 2.5 and 4.0 Average molecular weight should be from 3600 to 5000. Percentage of factions with molecular weight of less than 2000 should be not more than 15%; 2000-8000: 75-95%; 2000-4000: 35-55% White crystalline precipitate appears. |
|
Clarity of solution |
5% substance solution should stand comparison with the Reference II |
|
Color of solution |
5% substance solution should stand comparison with the Reference Y5 |
|
pH |
From 5.5 to 8.0 (1% solution) |
|
Free sulphates |
Not more than 0.5% |
|
N-NO groups |
maximum 0.25 ppm |
|
Calcium |
From 9.5 to 11.5% |
|
Nitrogen |
From 1.5 to 2.5% (calculated on the dried basis) |
|
Sulphate ion to carboxylate ion molecular ratio |
Not less than 1.8 |
|
Heavy metals |
Not more than 30 ppm |
|
Ethanol |
Not more than 1.0 per cent m/m |
|
Assay |
Anti-factor Xa activity should be from 95 to 130 IU/mg calculated on the dried basis. Anti-factor Xa activity to anti-factor IIa activity ratio should be from 2.5 to 4.0 |

Product detail pictures:
Related Product Guide:
Science, simplified:How vaccines work against COVID-19
We not only will try our greatest to offer you excellent services to just about every client, but also are ready to receive any suggestion offered by our buyers for 2020 China New Design Heparin Sodium Solution - Nadroparin Calcium – CSBIO , The product will supply to all over the world, such as: Greece, Albania, Costa rica, Why we can do these? Because: A, We are honest and reliable. Our products have high quality, attractive price, sufficient supply capacity and perfect service. B, Our geographical position has a big advantage . C, Various types: Welcome your inquiry, It will be highly appreciated.
We feel easy to cooperate with this company, the supplier is very responsible, thanks.There will be more in-depth cooperation.






